Experiment 2
Did Ms. Crowler spill the suckleberry soda?
Watson observes a puddle of water with raisin-like fruit rising and sinking in it. He wants to know if the water was from carbonated suckelberry soda, or the pond. This experiment will help him determine if Ms Crowler was telling the truth by observing and comparing the behavior of raisins submerged in tap water with raisins submerged in carbonated water (soft drink) over time.
I. Hypothesis Bubbles in the carbonated water will cause wrinkled fruit such as suckelberries
or raisins to rise and fall (dance).
II. Materials needed
• a can or bottle of carbonated water or clear soda (fizzy drink) like 7-Up™ or Sprite™.
• 2 tall, clear glasses or plastic cups
• 12 or more raisins (fresh raisins work the best)
• a glass of tap water
III. Procedure/Observation
-
Fill one glass with tap water and fill the other glass with carbonated
water or clear soda.
-
Drop 6 or 7 raisins into the glass of tap water and drop six or seven raisins
into the glass of carbonated water or soda. -
Watch the raisins for a few seconds. Describe what is happening to the raisins.
Do they sink or float? Do you notice any difference in how the raisins
behave in the tap compared to the carbonated water or soda? Keep watching.
What happens in the next several minutes?
IV] Detective Conclusions:
After observing how raisins behave in carbonated water, and reviewing what other detectives had observed, do you now know if Ms Crowly was telling the truth? Was the liquid in the puddle carbonated?
V] Further Exploration
Where do the bubbles come from?
Carbonated beverages are prepared by putting the beverage into a can or bottle under high pressure with carbon dioxide gas. The high pressure causes the carbon dioxide gas to dissolve in the liquid. When the can or bottle is opened the pressure inside the can decreases allowing some of the carbon dioxide gas dissolved in the liquid to escape as bubbles.
Why do the raisins rise?
A raisin’s density is slightly more than water and therefore the fruit will sink. When a raisin is placed in carbonated water, it will initially sink to the bottom. However, once the raisin settles, folds in its skin “catch” some of the bubbles that are released in the carbonated water. The density of the carbon dioxide in the bubbles is much lower than the density of water. Eventually enough bubbles will adhere to the raisin so the combined density of the raisin and bubbles is less than the surrounding water and the raisin will rise.
Why do the raisins fall?
When the raisin covered with bubbles reaches the surface, many of the bubbles break, releasing their carbon dioxide. With less bubbles on the raisin, the raisin’s density increases. When the raisin loses enough bubbles, so its overall density is greater than the surrounding water, it will sink.
VI] Relating to the Environment
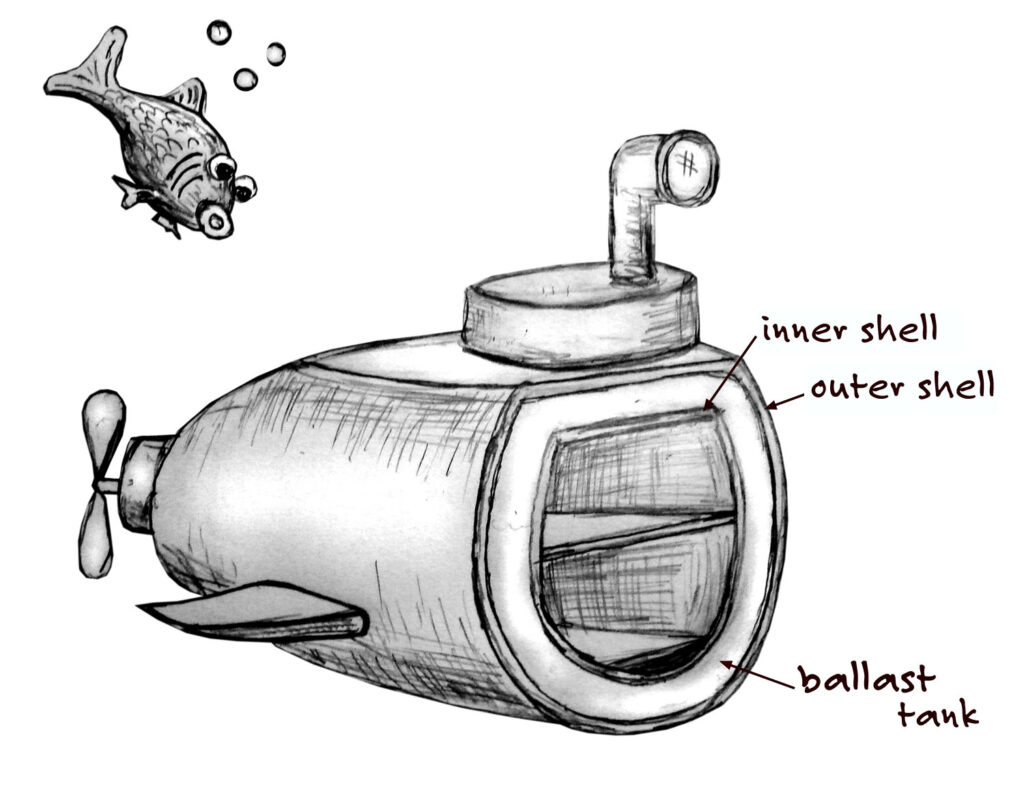
Submarines don’t attach bubbles to their sides to rise or sink. Instead, submarines have an inner and an outer steel shell, called a hull. The space in between the two hulls is called the ballast tank. It can be filled with either air or water. When the submarine is floating on the surface, the ballast tanks are filled with air and the submarine’s overall density is less than that of the surrounding water.
To dive, the submarine crew opens up valves to let air out of the ballast tanks. It’s like the bubbles on the raisin popping. Seawater rushes in to fill the space that was taken up by air. This increases the ship’s density. When the density of the submarine is greater than the surrounding water, it begins to sink. This is called negative buoyancy.